
- SAP Community
- Products and Technology
- Technology
- Technology Blogs by Members
- EUDAMED UDI records maintenance with SAP MDG Custo...
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content

Picture 1 - Implementation results
First, a few words about UDI:
Unique Device Identification (UDI) helps identifying medical products and their published master data in public databases. In short, if medical device producer wants to sell a product on some market (US, EU, UK, Korea, etc.) the product must be registered in corresponding country/union database. The databases can be accessed by anyone and most attributes of the registered medical devices can be found there (some data is registered but not openly published).
EU (EUDAMED):
https://webgate.ec.europa.eu/eudamed-help/?lang=en
US (GUDID):
https://www.fda.gov/medical-devices/unique-device-identification-system-udi-system/global-unique-dev...
Australia (AsuUDID):
https://www.tga.gov.au/how-we-regulate/manufacturing/manufacture-medical-device/unique-device-identi...
The attributes to be registered depend on country/organization requirements and in practice some of them are common across the databases, some values can be mapped between them, and others are specific for the particular database implementation. So, the idea was to create “Common Device Record” which is then used as base when publishing the device in given target database (by enhancing and mapping the values to specific external database requirements).
The whole architecture looks like below and in this blog I will just focus on the highlighted part:

Picture 2 - UDI-DI data architecture
Attributes of the medical devices does not match SAP material master fields (apart from material number and EAN), moreover the relation between material master and UDI is not 1:1 but rather 1:n (like material to EAN relation), so instead of extending already heavy MDG-MM model the solution has been implemented with MDG Custom Objects.
MDG Implementation overview
Data Model
As shown in Picture 2 the UDI records are maintained on different levels (Central record, GUDID UDI, EUDAMED UDI, EUDAMED Basic-UDI) logically they are all Medical Device data records, therefore a single custom MDG model has been used for all of them with several entities of type 1 (Business Objects). To have better control and easier access to active area the “Reuse” option has been used.
In case of EUDAMED records we have two kinds of Type 1 entities: “Basic UDI-DI records” (BUDI) and “UDI-DI records” (UDI-DI). In both cases they have some related sub-tables and lookup tables (entities of type 4 and 3).
Quick explanation: BUDI records (typical for EUDAMED) are grouping UDI-DI records (relation 1:n) – more details about that you can find on EUDAMED website.
Search and single record display
Even though there was no need to create workflow for single record processing the SP configuration still needed to be done – it is used for the records search and display.

Picture 3 - Records search
The search result list has been enhanced with current record status and synchronization icon (showing state of record replication to EUDAMED database)
The EUDAMED UDI-DI records differ depending on “applied legislation” (which is an attribute of linked Basic UDI-DI), that means for one type some of the fields are relevant and for the other type not. While data model was prepared to hold all the attributes, when displaying a single Medical Device record just the relevant fields should be shown.
That’s the right place for implementation of Context Based Adaptation (CBA), in result the records are shown with layout configured for assigned legislation. Below a sample view of Legacy Device (MDD legislation) and Regulation Device (MDR) record:

Picture 4 - Records details with CBA applied
And the MDG change request CBA context enhanced with the new dimension:
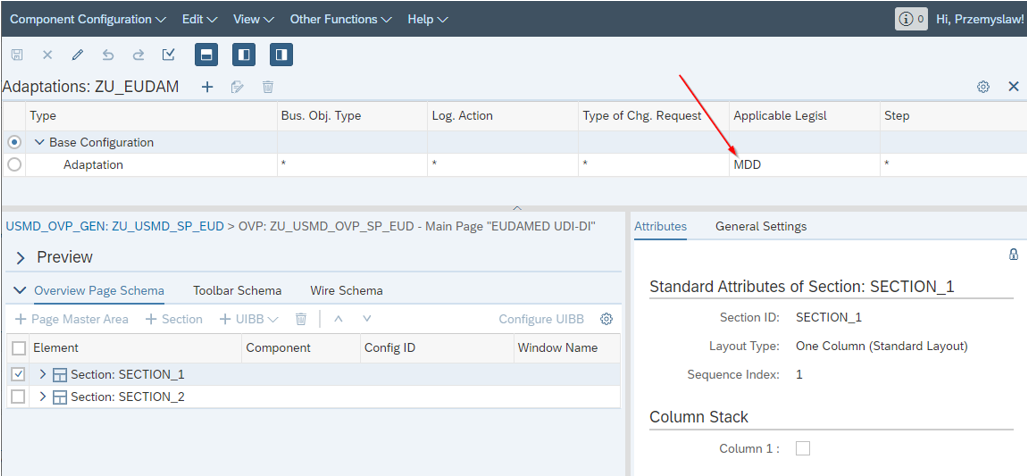
Picture 5 - CBA context with new dimension
The CBA context is set in application controller, not in the UIBB feeder class like suggested in CBA WIKI tutorial – the second option does not work correctly with “lazy loading”.
I had some fun with modeling multilingual fields of the UDI-DI records. In EUDAMED the texts are sometimes translatable sometimes not. The interesting attribute is “Trade Name”:

Picture 6 - EUDAMED Business Rule for multiple languages
Which means the language specific texts in this case are not linked to each other (they are not translations but just collection of different texts in multiple languages). I don’t know how such texts are stored and updated on EUDAMED side but “locally” there was made assumption that for given device there will be just one or 0 particular text per language.
Technically all the texts in (reuse) active area are stored in single texts table (with GUID and language as a key), however in MDG model they are split into separate entities depending on the text type. To display the translations, buttons have been added to the UI, they trigger popups with details:

Picture 7 - Translations pop-up
The buttons are hidden dynamically in case there are no texts or just English translation exists.
Additionally, there is a link created for BUDI record – it opens related BUDI details:

Picture 8 - Link to BUDI record
Multi record configuration
As mentioned at the beginning it was required to implement multi-record change request with highlighted changes. Fortunately, all the dialog tasks are just approvals (read-only mode) that eliminated the need of enhancing the UIBBs with add/delete functions – which would be a bit tricky especially for multilingual texts – anyway the FPM configuration of all UIBBs works on wires and standard MDG wire classes – so it is “edit-mode ready”.

Picture 9 - Multi-record Change Request with highlighted changes
As you can notice above, for new record there is temporary number generated (starting with $) it is replaced later with final record ID in access class during activation. Like in single processing also here have been implemented “dynamic buttons” for displaying multilingual texts details. All the UIBBs use common feeder class inheriting from standard CL_USMD_MC_FEEDER_LIST where just the three methods below have been redefined:

Picture 10 - Overridden inherited methods of UIBB feeder class
As the dialog steps are read-only all the validations and derivations have been implemented in “starting application” described below.
Records maintenance application
The logic of this application is simple:
- Export selected existing EUDAMED data and/or data to be created from “Common Device Record” into excel,
- After adjustments import the same excel file (showing changes in colors)
- Run validations and trigger new Change Requests with requested changes
This is achieved with the first three buttons below, additionally the last (toggle) button changes displaying mode of sub-tables

Picture 11 - Action buttons of maintenance application
For export/import of XLSX files the standard ABAP classes have been used: CL_CMCB_EXCEL_2007 and CL_FDT_XL_SPREADSHEET. The first one - for exporting - has rather limited formatting options (still they allow to create multiple sheets and, and use of four cell formatting styles), so to achieve really nice looking XLSX something as abap2xlsx library should be used, but for the project the CL_CMCB_EXCEL_2007 was sufficient.
As mentioned before, all the validations are triggered in the "maintenance application" because later the data in the workflow is read-only. Like in the workflow steps the changes are highlighted in yellow, additionally key fields are in blue and "to be deleted" rows are marked with red color.

Picture 12 - Validations results and highlighted changes in maintenance application
Another requirement was that potential processors assigned to corresponding validation/approval workflow tasks should be selected by the CR creator (from users with an assigned corresponding role). This, together with 4-eyes principle check has been implemented with collapsible list like below.

Picture 13 - Submit pop-up with potential processors assignment
Performance
SAP MDG Central Governance is known from performance issues, (especially MDG-M for materials with many Plants/Sales Orgs.). Here the model is no so complex, however we have multi-record CRs.
Below are shown time measurement results of three operations (MDG 9.2 on Hana DB - test system):
- Upload of the data from XLSX (all validations are triggered here)
- Submit of new CR from the “Record maintenance application” (this is done with MDG GOV API)
- Opening the created CR from “My Change Requests” (all UIBBs expanded)

Picture 14 - Performance measurement results
CRs with more than 400 records caused time outs when opened.
Below CR with 376 records:

Picture 15 - Stress test CR with many records
Conclusions
MDG Custom Objects can be successfully used to process multi-record change requests for relatively complex objects. Features like highlighting of changes, context based adaptations with enhanced dimensions or wiring UIBBs work as well pretty good.
There are some performance issues, however up to 100 objects in CR the lags are acceptable.
With custom coding a “maintenance application” can be created which exports/imports data to/from XLSX file, validates the imported data and triggers MDG CR. This requires some development effort but once done it can be easily used as template for another MDG Custom Objects implementations.
- SAP Managed Tags:
- SAP Master Data Governance
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.
-
"automatische backups"
1 -
"regelmäßige sicherung"
1 -
"TypeScript" "Development" "FeedBack"
1 -
505 Technology Updates 53
1 -
ABAP
14 -
ABAP API
1 -
ABAP CDS Views
2 -
ABAP CDS Views - BW Extraction
1 -
ABAP CDS Views - CDC (Change Data Capture)
1 -
ABAP class
2 -
ABAP Cloud
2 -
ABAP Development
5 -
ABAP in Eclipse
1 -
ABAP Platform Trial
1 -
ABAP Programming
2 -
abap technical
1 -
absl
1 -
access data from SAP Datasphere directly from Snowflake
1 -
Access data from SAP datasphere to Qliksense
1 -
Accrual
1 -
action
1 -
adapter modules
1 -
Addon
1 -
Adobe Document Services
1 -
ADS
1 -
ADS Config
1 -
ADS with ABAP
1 -
ADS with Java
1 -
ADT
2 -
Advance Shipping and Receiving
1 -
Advanced Event Mesh
3 -
AEM
1 -
AI
7 -
AI Launchpad
1 -
AI Projects
1 -
AIML
9 -
Alert in Sap analytical cloud
1 -
Amazon S3
1 -
Analytical Dataset
1 -
Analytical Model
1 -
Analytics
1 -
Analyze Workload Data
1 -
annotations
1 -
API
1 -
API and Integration
3 -
API Call
2 -
Application Architecture
1 -
Application Development
5 -
Application Development for SAP HANA Cloud
3 -
Applications and Business Processes (AP)
1 -
Artificial Intelligence
1 -
Artificial Intelligence (AI)
4 -
Artificial Intelligence (AI) 1 Business Trends 363 Business Trends 8 Digital Transformation with Cloud ERP (DT) 1 Event Information 462 Event Information 15 Expert Insights 114 Expert Insights 76 Life at SAP 418 Life at SAP 1 Product Updates 4
1 -
Artificial Intelligence (AI) blockchain Data & Analytics
1 -
Artificial Intelligence (AI) blockchain Data & Analytics Intelligent Enterprise
1 -
Artificial Intelligence (AI) blockchain Data & Analytics Intelligent Enterprise Oil Gas IoT Exploration Production
1 -
Artificial Intelligence (AI) blockchain Data & Analytics Intelligent Enterprise sustainability responsibility esg social compliance cybersecurity risk
1 -
ASE
1 -
ASR
2 -
ASUG
1 -
Attachments
1 -
Authorisations
1 -
Automating Processes
1 -
Automation
1 -
aws
2 -
Azure
1 -
Azure AI Studio
1 -
B2B Integration
1 -
Backorder Processing
1 -
Backup
1 -
Backup and Recovery
1 -
Backup schedule
1 -
BADI_MATERIAL_CHECK error message
1 -
Bank
1 -
BAS
1 -
basis
2 -
Basis Monitoring & Tcodes with Key notes
2 -
Batch Management
1 -
BDC
1 -
Best Practice
1 -
bitcoin
1 -
Blockchain
3 -
BOP in aATP
1 -
BOP Segments
1 -
BOP Strategies
1 -
BOP Variant
1 -
BPC
1 -
BPC LIVE
1 -
BTP
11 -
BTP Destination
2 -
Business AI
1 -
Business and IT Integration
1 -
Business application stu
1 -
Business Application Studio
1 -
Business Architecture
1 -
Business Communication Services
1 -
Business Continuity
1 -
Business Data Fabric
3 -
Business Partner
12 -
Business Partner Master Data
10 -
Business Technology Platform
2 -
Business Trends
1 -
CA
1 -
calculation view
1 -
CAP
3 -
Capgemini
1 -
CAPM
1 -
Catalyst for Efficiency: Revolutionizing SAP Integration Suite with Artificial Intelligence (AI) and
1 -
CCMS
2 -
CDQ
12 -
CDS
2 -
Cental Finance
1 -
Certificates
1 -
CFL
1 -
Change Management
1 -
chatbot
1 -
chatgpt
3 -
CL_SALV_TABLE
2 -
Class Runner
1 -
Classrunner
1 -
Cloud ALM Monitoring
1 -
Cloud ALM Operations
1 -
cloud connector
1 -
Cloud Extensibility
1 -
Cloud Foundry
4 -
Cloud Integration
6 -
Cloud Platform Integration
2 -
cloudalm
1 -
communication
1 -
Compensation Information Management
1 -
Compensation Management
1 -
Compliance
1 -
Compound Employee API
1 -
Configuration
1 -
Connectors
1 -
Consolidation Extension for SAP Analytics Cloud
1 -
Controller-Service-Repository pattern
1 -
Conversion
1 -
Cosine similarity
1 -
cryptocurrency
1 -
CSI
1 -
ctms
1 -
Custom chatbot
3 -
Custom Destination Service
1 -
custom fields
1 -
Customer Experience
1 -
Customer Journey
1 -
Customizing
1 -
Cyber Security
2 -
Data
1 -
Data & Analytics
1 -
Data Aging
1 -
Data Analytics
2 -
Data and Analytics (DA)
1 -
Data Archiving
1 -
Data Back-up
1 -
Data Governance
5 -
Data Integration
2 -
Data Quality
12 -
Data Quality Management
12 -
Data Synchronization
1 -
data transfer
1 -
Data Unleashed
1 -
Data Value
8 -
database tables
1 -
Datasphere
2 -
datenbanksicherung
1 -
dba cockpit
1 -
dbacockpit
1 -
Debugging
2 -
Delimiting Pay Components
1 -
Delta Integrations
1 -
Destination
3 -
Destination Service
1 -
Developer extensibility
1 -
Developing with SAP Integration Suite
1 -
Devops
1 -
digital transformation
1 -
Documentation
1 -
Dot Product
1 -
DQM
1 -
dump database
1 -
dump transaction
1 -
e-Invoice
1 -
E4H Conversion
1 -
Eclipse ADT ABAP Development Tools
2 -
edoc
1 -
edocument
1 -
ELA
1 -
Embedded Consolidation
1 -
Embedding
1 -
Embeddings
1 -
Employee Central
1 -
Employee Central Payroll
1 -
Employee Central Time Off
1 -
Employee Information
1 -
Employee Rehires
1 -
Enable Now
1 -
Enable now manager
1 -
endpoint
1 -
Enhancement Request
1 -
Enterprise Architecture
1 -
ETL Business Analytics with SAP Signavio
1 -
Euclidean distance
1 -
Event Dates
1 -
Event Driven Architecture
1 -
Event Mesh
2 -
Event Reason
1 -
EventBasedIntegration
1 -
EWM
1 -
EWM Outbound configuration
1 -
EWM-TM-Integration
1 -
Existing Event Changes
1 -
Expand
1 -
Expert
2 -
Expert Insights
1 -
Fiori
14 -
Fiori Elements
2 -
Fiori SAPUI5
12 -
Flask
1 -
Full Stack
8 -
Funds Management
1 -
General
1 -
Generative AI
1 -
Getting Started
1 -
GitHub
8 -
Grants Management
1 -
groovy
1 -
GTP
1 -
HANA
5 -
HANA Cloud
2 -
Hana Cloud Database Integration
2 -
HANA DB
1 -
HANA XS Advanced
1 -
Historical Events
1 -
home labs
1 -
HowTo
1 -
HR Data Management
1 -
html5
8 -
HTML5 Application
1 -
Identity cards validation
1 -
idm
1 -
Implementation
1 -
input parameter
1 -
instant payments
1 -
Integration
3 -
Integration Advisor
1 -
Integration Architecture
1 -
Integration Center
1 -
Integration Suite
1 -
intelligent enterprise
1 -
Java
1 -
job
1 -
Job Information Changes
1 -
Job-Related Events
1 -
Job_Event_Information
1 -
joule
4 -
Journal Entries
1 -
Just Ask
1 -
Kerberos for ABAP
8 -
Kerberos for JAVA
8 -
Launch Wizard
1 -
Learning Content
2 -
Life at SAP
1 -
lightning
1 -
Linear Regression SAP HANA Cloud
1 -
local tax regulations
1 -
LP
1 -
Machine Learning
2 -
Marketing
1 -
Master Data
3 -
Master Data Management
14 -
Maxdb
2 -
MDG
1 -
MDGM
1 -
MDM
1 -
Message box.
1 -
Messages on RF Device
1 -
Microservices Architecture
1 -
Microsoft Universal Print
1 -
Middleware Solutions
1 -
Migration
5 -
ML Model Development
1 -
Modeling in SAP HANA Cloud
8 -
Monitoring
3 -
MTA
1 -
Multi-Record Scenarios
1 -
Multiple Event Triggers
1 -
Neo
1 -
New Event Creation
1 -
New Feature
1 -
Newcomer
1 -
NodeJS
2 -
ODATA
2 -
OData APIs
1 -
odatav2
1 -
ODATAV4
1 -
ODBC
1 -
ODBC Connection
1 -
Onpremise
1 -
open source
2 -
OpenAI API
1 -
Oracle
1 -
PaPM
1 -
PaPM Dynamic Data Copy through Writer function
1 -
PaPM Remote Call
1 -
PAS-C01
1 -
Pay Component Management
1 -
PGP
1 -
Pickle
1 -
PLANNING ARCHITECTURE
1 -
Popup in Sap analytical cloud
1 -
PostgrSQL
1 -
POSTMAN
1 -
Process Automation
2 -
Product Updates
4 -
PSM
1 -
Public Cloud
1 -
Python
4 -
Qlik
1 -
Qualtrics
1 -
RAP
3 -
RAP BO
2 -
Record Deletion
1 -
Recovery
1 -
recurring payments
1 -
redeply
1 -
Release
1 -
Remote Consumption Model
1 -
Replication Flows
1 -
Research
1 -
Resilience
1 -
REST
1 -
REST API
1 -
Retagging Required
1 -
Risk
1 -
Rolling Kernel Switch
1 -
route
1 -
rules
1 -
S4 HANA
1 -
S4 HANA Cloud
1 -
S4 HANA On-Premise
1 -
S4HANA
3 -
S4HANA_OP_2023
2 -
SAC
10 -
SAC PLANNING
9 -
SAP
4 -
SAP ABAP
1 -
SAP Advanced Event Mesh
1 -
SAP AI Core
8 -
SAP AI Launchpad
8 -
SAP Analytic Cloud Compass
1 -
Sap Analytical Cloud
1 -
SAP Analytics Cloud
4 -
SAP Analytics Cloud for Consolidation
2 -
SAP Analytics Cloud Story
1 -
SAP analytics clouds
1 -
SAP BAS
1 -
SAP Basis
6 -
SAP BODS
1 -
SAP BODS certification.
1 -
SAP BTP
20 -
SAP BTP Build Work Zone
2 -
SAP BTP Cloud Foundry
5 -
SAP BTP Costing
1 -
SAP BTP CTMS
1 -
SAP BTP Innovation
1 -
SAP BTP Migration Tool
1 -
SAP BTP SDK IOS
1 -
SAP Build
11 -
SAP Build App
1 -
SAP Build apps
1 -
SAP Build CodeJam
1 -
SAP Build Process Automation
3 -
SAP Build work zone
10 -
SAP Business Objects Platform
1 -
SAP Business Technology
2 -
SAP Business Technology Platform (XP)
1 -
sap bw
1 -
SAP CAP
2 -
SAP CDC
1 -
SAP CDP
1 -
SAP Certification
1 -
SAP Cloud ALM
4 -
SAP Cloud Application Programming Model
1 -
SAP Cloud Integration for Data Services
1 -
SAP cloud platform
8 -
SAP Companion
1 -
SAP CPI
3 -
SAP CPI (Cloud Platform Integration)
2 -
SAP CPI Discover tab
1 -
sap credential store
1 -
SAP Customer Data Cloud
1 -
SAP Customer Data Platform
1 -
SAP Data Intelligence
1 -
SAP Data Migration in Retail Industry
1 -
SAP Data Services
1 -
SAP DATABASE
1 -
SAP Dataspher to Non SAP BI tools
1 -
SAP Datasphere
9 -
SAP DRC
1 -
SAP EWM
1 -
SAP Fiori
2 -
SAP Fiori App Embedding
1 -
Sap Fiori Extension Project Using BAS
1 -
SAP GRC
1 -
SAP HANA
1 -
SAP HCM (Human Capital Management)
1 -
SAP HR Solutions
1 -
SAP IDM
1 -
SAP Integration Suite
9 -
SAP Integrations
4 -
SAP iRPA
2 -
SAP Learning Class
1 -
SAP Learning Hub
1 -
SAP Odata
2 -
SAP on Azure
1 -
SAP PartnerEdge
1 -
sap partners
1 -
SAP Password Reset
1 -
SAP PO Migration
1 -
SAP Prepackaged Content
1 -
SAP Process Automation
2 -
SAP Process Integration
2 -
SAP Process Orchestration
1 -
SAP S4HANA
2 -
SAP S4HANA Cloud
1 -
SAP S4HANA Cloud for Finance
1 -
SAP S4HANA Cloud private edition
1 -
SAP Sandbox
1 -
SAP STMS
1 -
SAP SuccessFactors
2 -
SAP SuccessFactors HXM Core
1 -
SAP Time
1 -
SAP TM
2 -
SAP Trading Partner Management
1 -
SAP UI5
1 -
SAP Upgrade
1 -
SAP-GUI
8 -
SAP_COM_0276
1 -
SAPBTP
1 -
SAPCPI
1 -
SAPEWM
1 -
sapmentors
1 -
saponaws
2 -
SAPUI5
4 -
schedule
1 -
Secure Login Client Setup
8 -
security
9 -
Selenium Testing
1 -
SEN
1 -
SEN Manager
1 -
service
1 -
SET_CELL_TYPE
1 -
SET_CELL_TYPE_COLUMN
1 -
SFTP scenario
2 -
Simplex
1 -
Single Sign On
8 -
Singlesource
1 -
SKLearn
1 -
soap
1 -
Software Development
1 -
SOLMAN
1 -
solman 7.2
2 -
Solution Manager
3 -
sp_dumpdb
1 -
sp_dumptrans
1 -
SQL
1 -
sql script
1 -
SSL
8 -
SSO
8 -
Substring function
1 -
SuccessFactors
1 -
SuccessFactors Time Tracking
1 -
Sybase
1 -
system copy method
1 -
System owner
1 -
Table splitting
1 -
Tax Integration
1 -
Technical article
1 -
Technical articles
1 -
Technology Updates
1 -
Technology Updates
1 -
Technology_Updates
1 -
Threats
1 -
Time Collectors
1 -
Time Off
2 -
Tips and tricks
2 -
Tools
1 -
Trainings & Certifications
1 -
Transport in SAP BODS
1 -
Transport Management
1 -
TypeScript
2 -
unbind
1 -
Unified Customer Profile
1 -
UPB
1 -
Use of Parameters for Data Copy in PaPM
1 -
User Unlock
1 -
VA02
1 -
Validations
1 -
Vector Database
1 -
Vector Engine
1 -
Visual Studio Code
1 -
VSCode
1 -
Web SDK
1 -
work zone
1 -
workload
1 -
xsa
1 -
XSA Refresh
1
- « Previous
- Next »
- Empowering Retail Business with a Seamless Data Migration to SAP S/4HANA in Technology Blogs by Members
- 10+ ways to reshape your SAP landscape with SAP Business Technology Platform - Blog 7 in Technology Blogs by SAP
- Data Flows - The Python Script Operator and why you should avoid it in Technology Blogs by Members
- S/4HANA 2023 FPS00 Upgrade in Technology Blogs by Members
- Handle Special Character(Single Quote) in Master Data Loading in Technology Q&A
| User | Count |
|---|---|
| 11 | |
| 10 | |
| 7 | |
| 6 | |
| 4 | |
| 4 | |
| 3 | |
| 3 | |
| 3 | |
| 3 |